Abstract
Background. Despite advances in targeted and cellular therapy, outcomes among patients with RT and tNHL remain dismal. Copanlisib (COPA) is a selective, small molecule, PI3K inhibitor which preferentially targets the p110αδ isoforms. COPA has shown clinical efficacy in NHL and is an approved therapy for follicular lymphoma (FL). Nivolumab is a PD-1 antagonist which has demonstrated activity in Hodgkin lymphoma as well as NHL. Furthermore, combined targeting of PI3K and PD-1 has demonstrated synergy in pre-clinical lymphoma models. Here we report initial results of a phase 1 study of COPA in combination with nivolumab in patients with R/R RT or tNHL (NCT03884998).
Methods. This ongoing multicenter, open-label, phase I, investigator-sponsored study is enrolling patients with RT or tNHL, age ≥18 years, whose disease has relapsed or was refractory to ≥1 prior line of therapy. The phase I portion of the study followed a standard 3+3 design, followed by a dose expansion of 10 evaluable patients, with three planned dose levels of COPA administered IV (dose level [DL]1 - 45 mg on days 1, 8 and 15; DL2 - 60 mg on days 1, 8 and 15; DL "-1" - 45 mg on days 1 and 15 of a 28-day cycle). Nivolumab 240 mg was given IV on days 1 and 15. Patients received up to 24 cycles of therapy. The primary study objective was to evaluate the maximum tolerated dose (MTD) of the combination; secondary objectives included preliminary measures of efficacy by Lugano criteria including PET/CT scans. Exploratory objectives included pharmacodynamic endpoints. Dose limiting toxicities (DLT) were defined as grade ≥4 hematologic > 7 days or grade ≥3 non-hematologic toxicities.
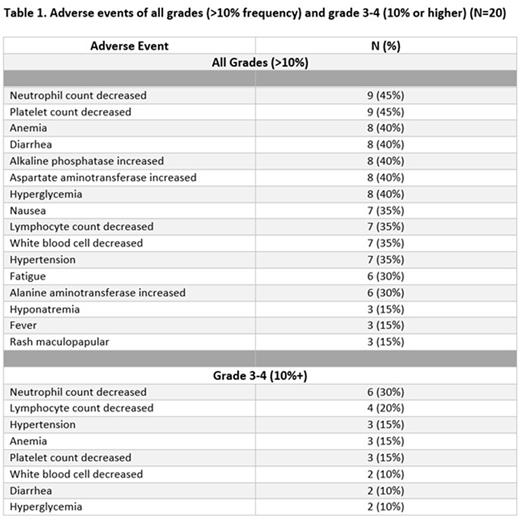
Results. Twenty patients have been enrolled, 11 for dose finding (8 at DL1 and 3 at DL2) and 9 for dose expansion. Thirteen had RT and 7 had tNHL (6 FL and 1 lymphoplasmacytic lymphoma). Median age was 65 (32-77) years, 85% (17/20) had an ECOG performance status ≤1. Patients had received a median of 4 prior lines of therapy (range, 1-10), including 7 patients (2 RT, 5 tNHL) who had undergone CAR T cell therapy. Eight patients were treated at DL1 (45 mg COPA), of which 2 patients did not complete the DLT period due to rapidly progressive disease and were replaced. No DLTs were observed in 6 evaluable patients at DL 1. At DL2 (60 mg COPA), three DLTs (grade 4 febrile neutropenia and grade 4 neutropenia in 1, grade 4 thrombocytopenia in another) were observed among the three patients treated. Therefore, the MTD and recommended phase 2 dose (RP2D) of COPA in combination with nivolumab was determined to be 45 mg on days 1, 8, 15. The most common treatment-related adverse events (AEs, any grade) were thrombocytopenia and neutropenia (Table 1). Eighteen patients went off protocol after a median of 2 cycles (range 1-20); 2 remain on treatment. Twelve pts discontinued therapy due to progressive disease and 6 due to AEs. Among the 17 efficacy-evaluable patients who completed at least one cycle of therapy, 8 patients achieved a response per the investigators' assessment (ORR 47%). Patients with tFL had an ORR of 100% (2 CR and 3 PR). Patients with RT had an ORR of 27% (1 CR and 2 PR). Efficacy assessment is ongoing.
Discussion. We identified COPA 45 mg IV on days 1, 8 and 15 as the MTD/RP2D in combination with nivolumab in patients with R/R RT and tFL. This combination was well-tolerated at the MTD/RP2D, and the study is currently enrolling patients in an expansion cohort to further characterize the efficacy of this regimen.
Disclosures
Shouse:Kite Pharma: Speakers Bureau; Beigene Inc USA: Honoraria. Siddiqi:TG Therapeutics: Research Funding; Oncternal: Research Funding; Seattle Genetics: Speakers Bureau; Janssen: Speakers Bureau; PCYC: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Kittai:Abbvie: Consultancy; Astrazeneca: Consultancy, Research Funding; Beigene: Consultancy; Janssen: Consultancy. Davids:Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ascentage Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Consultancy, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ono Pharmaceuticals: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly and Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy; Novartis: Research Funding; Takeda: Consultancy; Research to Practice: Honoraria; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Research Funding. Danilov:Pharmacyclics: Consultancy; GSK: Consultancy; Takeda Oncology: Research Funding; Astra Zeneca: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Nurix: Consultancy, Research Funding; Cyclacel: Research Funding; MEI: Consultancy, Research Funding; Morphosys: Consultancy; Bayer Oncology: Research Funding; Bristol-Meyers-Squibb: Consultancy, Research Funding; Beigene: Consultancy; Genentech: Consultancy; Incyte: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal